Appendix#
Point group flowchart#
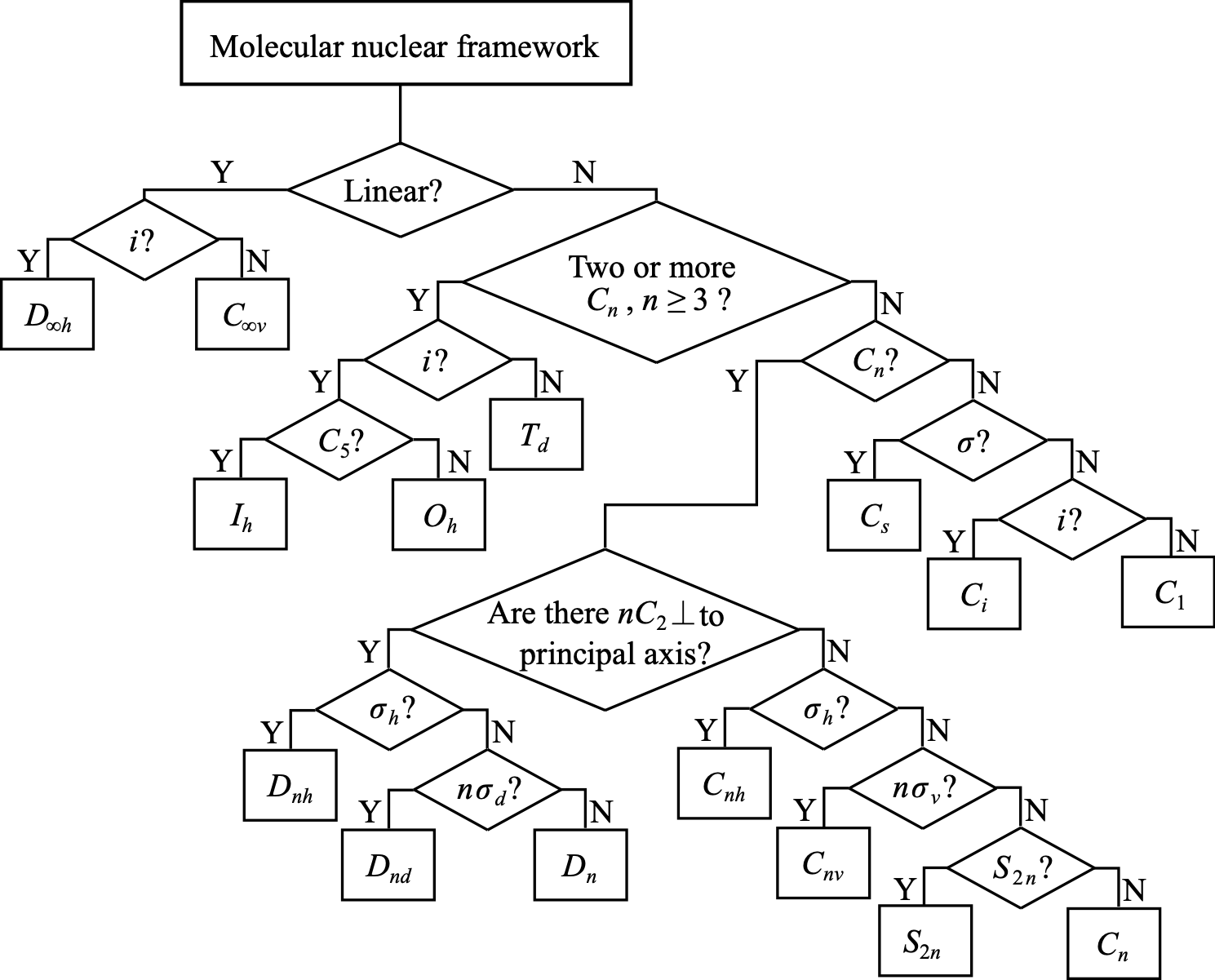
Fig. 1 Flowchart for the determination of molecular point groups.#
Character tables#
C\(_{2h}\)#
Irrep |
\(\hat{E}\) |
\(\hat{C}_2(z)\) |
\(\hat{i}\) |
\(\hat{\sigma}_h\) |
Operation |
|---|---|---|---|---|---|
\(A_g\) |
1 |
1 |
1 |
1 |
\(R_z\), \(x^2\), \(y^2\), \(z^2\) |
\(B_{g}\) |
1 |
-1 |
1 |
-1 |
\(R_x\), \(R_y\) |
\(A_{u}\) |
1 |
1 |
-1 |
-1 |
\(z\) |
\(B_{u}\) |
1 |
-1 |
-1 |
1 |
\(x\), \(y\) |
D\(_{2h}\)#
Irrep |
\(\hat{E}\) |
\(\hat{C}_2(z)\) |
\(\hat{C}_2(y)\) |
\(\hat{C}_2(x)\) |
\(\hat{i}\) |
\(\hat{\sigma}(xy)\) |
\(\hat{\sigma}(xz)\) |
\(\hat{\sigma}(yz)\) |
Operation |
|---|---|---|---|---|---|---|---|---|---|
\(A_g\) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
\(x^2\), \(y^2\), \(z^2\) |
\(B_{1g}\) |
1 |
1 |
-1 |
-1 |
1 |
1 |
-1 |
-1 |
\(R_z\), \(xy\) |
\(B_{2g}\) |
1 |
-1 |
1 |
-1 |
1 |
-1 |
1 |
-1 |
\(R_y\), \(xz\) |
\(B_{3g}\) |
1 |
-1 |
-1 |
1 |
1 |
-1 |
-1 |
1 |
\(R_x\), \(yz\) |
\(A_{u}\) |
1 |
1 |
1 |
1 |
-1 |
-1 |
-1 |
-1 |
|
\(B_{1u}\) |
1 |
1 |
-1 |
-1 |
-1 |
-1 |
1 |
1 |
\(z\) |
\(B_{2u}\) |
1 |
-1 |
1 |
-1 |
-1 |
1 |
-1 |
1 |
\(y\) |
\(B_{3u}\) |
1 |
-1 |
-1 |
1 |
-1 |
1 |
1 |
-1 |
\(x\) |
Atomic units#
A central problem in molecular physics is to solve the time-independent Schrödinger equation for the electrons in the field of the nuclei. Most often atomic units are then adopted. For the hydrogen atom, we have
where \(\hbar\) is the reduced Planck constant, \(m_\mathrm{e}\) is the electron mass, \(e\) is the elementary charge, and \(\varepsilon_0\) is the electric constant. To cast this equation into dimensionless form, consider a coordinate transformation of the form
to arrive at
Choose \(\lambda\) so that
with the solution
With \(E' = E/E_h\), we get
with a solution for the ground state energy that is equal to \(E' = -0.5\) a.u. (or Hartree). The defined quantity \(a_0\) is equal to the Bohr radius and the atomic unit of length is therefore also referred to as Bohr.
Table: Atomic unit conversion factors.
Quantity |
Symbol |
Atomic unit |
SI equivalent |
|---|---|---|---|
Energy |
\(E\) |
1 \(E_\mathrm{h}\) |
4.359 744\(\times 10^{-18}\) J |
Reduced Planck constant |
\(h = 2\pi\hbar\) |
1 \(\hbar\) |
1.054 572\(\times 10^{-34}\) J s |
Time |
\(t\) |
1 \(\hbar E_\mathrm{h}^{-1}\) |
2.418 884\(\times 10^{-17}\) s |
Length |
\(l\) |
1 \(a_0\) |
5.291 772\(\times 10^{-11}\) m |
Speed of light |
\(c\) |
137.036 \(a_0 E_h \hbar^{-1}\) |
2.997 925\(\times 10^{8}\) m s\(^{-1}\) |
Electric constant |
\(\varepsilon_0\) |
1 \(4\pi\varepsilon_0\) |
8.854 188\(\times 10^{-12}\) F m\(^{-1}\) |
Fine structure constant |
\(\alpha\) |
1/137.036 \(e^2( a_0 E_h 4\pi\varepsilon_0)^{-1}\) |
7.297 353\(\times 10^{3}\) |
Charge |
\(q\) |
1 \(e\) |
1.602 176\(\times 10^{-19}\) C |
Electric field |
\(F\) |
1 \(E_h (e a_{0})^{-1}\) |
5.142 207\(\times 10^{11}\) V m\(^{-1}\) |
Dipole moment |
\(\mu\) |
1 \(e a_{0}\) |
8.478 353\(\times 10^{-30}\) C m |
Mass |
\(m\) |
1 \(m_e\) |
9.109 383\(\times 10^{-31}\) kg |
