Excited states#
For the analysis of the excited states, it is common to simply study the molecular orbitals of the final states, and base assignments and other conclusions from this. This often works quite well, but the final state can be multi-determinational in nature, and the canonical orbitals of larger systems are often quite delocalized and hard to study. As such, many other analysis tools have been developed, two of which will be discussed here:
Natural transition orbitals (NTOs), which constructs a compact, transition-dependent description of specific excitations
Attachment and detachment densities, which are based on the one-particle density matrix and considers the density change involved in an excitation
As an illustration, we consider the first valence-excited state of water, as calculated using ADC(2):
import gator
water_mol_str = """
O 0.0000000000 0.1187290000 0.0000000000
H -0.7532010000 -0.4749160000 -0.0000000000
H 0.7532010000 -0.4749160000 0.0000000000
"""
# Construct structure and basis objects
molecule = gator.get_molecule(water_mol_str)
basis = gator.get_molecular_basis(molecule, "6-31G", ostream=None)
3Dmol.js failed to load for some reason. Please check your browser console for error messages.
# Perform SCF calculation
scf_gs = gator.run_scf(molecule, basis)
# Calculate the 2 lowest eigenstates
adc_results = gator.run_adc(molecule, basis, scf_gs, method="adc2", singlets=2)
Printing the results:
# Print results
print(adc_state.describe())
# Print dominant amplitudes
print(adc_state.describe_amplitudes())
+--------------------------------------------------------------+
| adc2 singlet , converged |
+--------------------------------------------------------------+
| # excitation energy osc str |v1|^2 |v2|^2 |
| (au) (eV) |
| 0 0.3054352 8.311314 0.0142 0.9591 0.04089 |
| 1 0.3903211 10.62118 0.0000 0.9636 0.03637 |
+--------------------------------------------------------------+
+---------------------------------------------------+
| State 0 , 0.3054352 au, 8.311314 eV |
+---------------------------------------------------+
| HOMO -> LUMO a ->a -0.691 |
| HOMO -> LUMO+4 a ->a +0.0451 |
| HOMO -> LUMO+7 a ->a +0.0227 |
| HOMO-2 HOMO -> LUMO+1 LUMO ab->ab +0.0306 |
| HOMO HOMO -> LUMO LUMO+3 ab->ab -0.0253 |
| HOMO-2 HOMO -> LUMO LUMO+1 aa->aa -0.0191 |
| HOMO-1 HOMO -> LUMO LUMO ab->ab -0.0191 |
| HOMO-3 HOMO -> LUMO LUMO ab->ab +0.0182 |
| HOMO-1 HOMO -> LUMO LUMO+4 aa->aa -0.0174 |
| HOMO-1 HOMO -> LUMO+4 LUMO ab->ab +0.0152 |
| HOMO-2 HOMO -> LUMO+6 LUMO ab->ab -0.0152 |
| HOMO-1 HOMO -> LUMO+5 LUMO ab->ab -0.0147 |
| HOMO-2 HOMO -> LUMO LUMO+6 aa->aa +0.0139 |
| HOMO-1 HOMO -> LUMO LUMO+5 aa->aa +0.0117 |
| HOMO-2 HOMO -> LUMO LUMO+1 ab->ab +0.0115 |
| HOMO-3 HOMO -> LUMO LUMO+7 aa->aa +0.0107 |
+---------------------------------------------------+
| State 1 , 0.3903211 au, 10.62118 eV |
+---------------------------------------------------+
| HOMO -> LUMO+1 a ->a +0.69 |
| HOMO -> LUMO+6 a ->a -0.0598 |
| HOMO -> LUMO+2 a ->a -0.0431 |
| HOMO-2 HOMO -> LUMO+1 LUMO+1 ab->ab -0.0301 |
| HOMO HOMO -> LUMO+1 LUMO+3 ab->ab +0.0234 |
| HOMO-1 HOMO -> LUMO LUMO+1 ab->ab +0.019 |
| HOMO-2 HOMO -> LUMO+1 LUMO+6 aa->aa -0.0189 |
| HOMO-1 HOMO -> LUMO+4 LUMO+1 ab->ab -0.0144 |
| HOMO-3 HOMO -> LUMO LUMO+1 ab->ab -0.0143 |
| HOMO-1 HOMO -> LUMO+1 LUMO+4 aa->aa +0.0133 |
| HOMO-2 HOMO -> LUMO+6 LUMO+1 ab->ab +0.013 |
| HOMO-1 HOMO -> LUMO+5 LUMO+1 ab->ab +0.0121 |
| HOMO-1 HOMO -> LUMO+1 LUMO+5 aa->aa -0.0109 |
| HOMO-1 HOMO -> LUMO LUMO+1 aa->aa +0.0104 |
Here we print the basic description of the excited states (energy, intensity, weight of double excitation amplitudes), as well as a break-down of the dominating amplitudes for each transition. From this we can start analysing the excited state by looking at the canonical MOs, but we will now use the results for a more involved analysis.
Natural transition orbitals#
Natural transition orbitals (NTOs) are constructed to provide the most compact, transition-dependent transition orbitals of a specific excitation. This is discussed in detail in the NTO section under spectra and properties, and we here illustrate the implementation in OrbitalViewer.
Calculating the first two excited states of water:
import veloxchem as vlx
water_mol_str = """
O 0.0000000000 0.1187290000 0.0000000000
H -0.7532010000 -0.4749160000 -0.0000000000
H 0.7532010000 -0.4749160000 0.0000000000
"""
# Construct structure and basis objects
molecule = vlx.Molecule.read_str(water_mol_str)
basis = vlx.MolecularBasis.read(molecule, "6-31G", ostream=None)
# SCF settings and calculation
scf_drv = vlx.ScfRestrictedDriver()
scf_drv.ostream.mute()
scf_drv.xcfun = "b3lyp"
scf_drv.filename = "vlx_vis"
scf_results = scf_drv.compute(molecule, basis)
# resolve four eigenstates
rpa_solver = vlx.lreigensolver.LinearResponseEigenSolver()
rpa_solver.nstates = 2
rpa_solver.nto = True
rpa_solver.nto_cubes = False
rpa_results = rpa_solver.compute(molecule, basis, scf_results)
The NTOs are plotted as :
viewer = vlx.OrbitalViewer()
viewer.plot(molecule, basis, "vlx_vis.h5", "rsp/NTO_S1")
Note
As this is an interactive viewer, it will not be able to change the MO to visualize in the compiled html-book. It will work in a notebook, though.
Attachment and detachment densities#
A different method for visualizing the transitions is to consider the attachment (A) and detachment (D) densities, which are constructed to show the density change related to an excitation [HGGMW95, PBapplerWD14, PWD14]. For a simple HOMO-LUMO transition they would simply correspond to the square of the dominant NTOs, with meaning:
Hole/detachment density representing where electrons go from
Electron/attachment density representing where electrons go to
These densities can then be used to look at properties such as hole and electron size, distance between the centroid (and thus level of charge-transfer), and more. D/A densities are constructed from the one-particle difference density matrix (1DDM), which is simply the difference between the one-particle density matrices of the initial and final state:
The sum of the attachment and detachment densities is then the difference density.
Diagonalizing 1DDM
The attachment and detachment densities are constructed by considering the negative and positive eigenvalues, and the trace of either is the number of electron involved in the excitation.
Looking at the attachment and detachment density of the first excited state:
# Load transition density matrix, combine alpha and beta, and transform to numpy
p_state = adc_state.state_diffdm[i].to_ao_basis()
p_state_ao = (p_state[0] + p_state[1]).to_ndarray()
# Diagonalize the 1DDM
k, w = np.linalg.eigh(p_state_ao)
k_detach = k.copy()
k_attach = k.copy()
# Detachment: set positive eigenvalues to 0
k_detach[k > 0] = 0
# Attachment: set negative eigenvalues to 0
k_attach[k < 0] = 0
# Back-transform with numpy
detach_ao = w @ np.diag(k_detach) @ w.T
attach_ao = w @ np.diag(k_attach) @ w.T
# Write cube-files
de = tools.cubegen.density(
mol, dm=detach_ao, outfile="../../img/visualize/water_detachment_{}.cube".format(i)
)
at = tools.cubegen.density(
mol, dm=attach_ao, outfile="../../img/visualize/water_attachment_{}.cube".format(i)
)
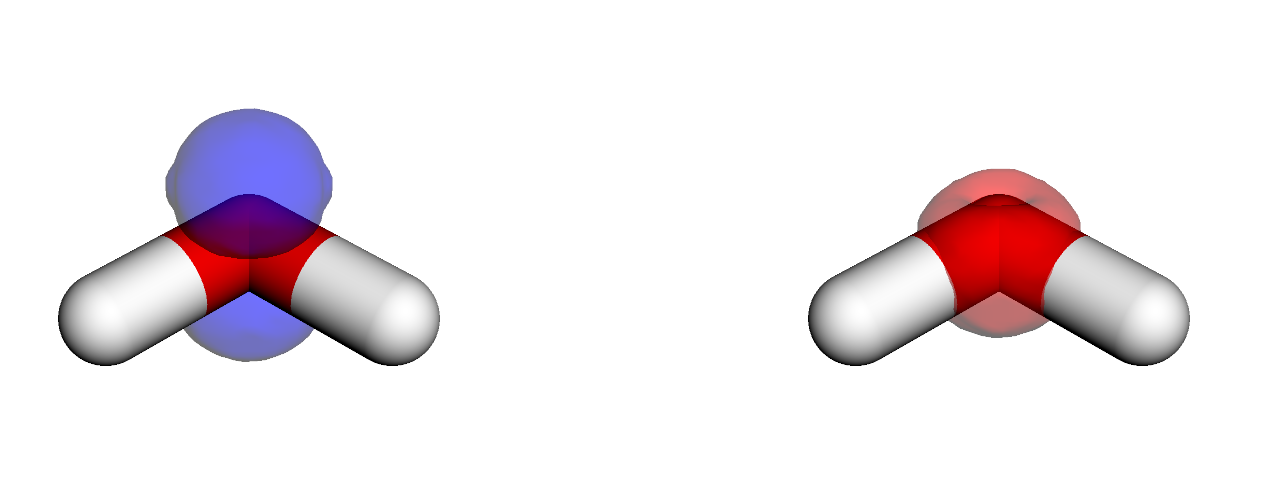
We see that these states are more compact (as we are looking on densities) than above NTOs. For a discussion on NTOs and D/A densities, see, e.g., this paper.
Visual analysis of electronic transitions#
The attachment and detachment densities (as well as other density types such as transition, electron, or hole densities) can be further analyzed quantitatively to determine electron localization and transfer between different parts of a molecular system. This is achieved by fragmenting the system into (chemically relevant) subgroups and performing a geometric segmentation of the selected density over these subgroups (using Voronoi segmentation). Charge transfer between molecular fragments can then be calculated. This is achieved by a constrained optimization algorithm since the problem does not have a unique solution. See the complete formulation here.
To illustrate this quantitative analysis method, we will analyze the nature of the excited states of the ethene (E) - tetrafluoroethene (TFE) dimer. This dimer has been used to illustrate the failures of TDDFT in the description of charge transfer (CT) states (see this article).
We start by constructing the dimer from the two monomers.
from valet import transition_analysis_utils as tau
import veloxchem as vlx
# Construct the dimer
c2h4_xyz = """
C 0.6655087212 0.0000000000 -x
C -0.6655087212 0.0000000000 -x
H 1.2112231877 0.9379948213 -x
H 1.2112231877 -0.9379948213 -x
H -1.2112231877 0.9379948213 -x
H -1.2112231877 -0.9379948213 -x
"""
c2f4_str = """
C 0.6665934397 0.0000000000 x
C -0.6665934397 -0.0000000000 x
F 1.3499511337 1.1743643195 x
F 1.3499511337 -1.1743643195 x
F -1.3499511337 1.1743643195 x
F -1.3499511337 -1.1743643195 x
"""
dist = 2 # We will set the interatomic distance to 4 Angstrom
dist_str = "%.10f" % dist
dimer_xyz = "12\n\n"
for line in c2h4_xyz.split("\n"):
new = line.replace('x', dist_str)
if 'x' in line:
dimer_xyz += new + "\n"
for line in c2f4_str.split("\n"):
new = line.replace('x', dist_str)
if 'x' in line:
dimer_xyz += new + "\n"
print(dimer_xyz)
molecule = vlx.Molecule.from_xyz_string(dimer_xyz)
basis_label = "6-31G*"
basis = vlx.MolecularBasis.read(molecule, basis_label, ostream=None)
12
C 0.6655087212 0.0000000000 -2.0000000000
C -0.6655087212 0.0000000000 -2.0000000000
H 1.2112231877 0.9379948213 -2.0000000000
H 1.2112231877 -0.9379948213 -2.0000000000
H -1.2112231877 0.9379948213 -2.0000000000
H -1.2112231877 -0.9379948213 -2.0000000000
C 0.6665934397 0.0000000000 2.0000000000
C -0.6665934397 -0.0000000000 2.0000000000
F 1.3499511337 1.1743643195 2.0000000000
F 1.3499511337 -1.1743643195 2.0000000000
F -1.3499511337 1.1743643195 2.0000000000
F -1.3499511337 -1.1743643195 2.0000000000
3Dmol.js failed to load for some reason. Please check your browser console for error messages.
# Next, we calculate the excited states at the B3LYP level of theory
method_dict = {'xcfun':'b3lyp'}
scf_dict = {}
scf_drv = vlx.ScfRestrictedDriver()
scf_drv.ostream.mute()
scf_drv.update_settings(scf_dict={}, method_dict=method_dict)
scf_results = scf_drv.compute(molecule, basis)
# We now run the excited states calculation and save the attachment and
# detachment densities in cube files
rsp_dict = {'detach_attach':'yes', 'nstates':5, 'filename':'dimer_4A'}
rsp_drv = vlx.lreigensolver.LinearResponseEigenSolver()
rsp_drv.update_settings(rsp_dict=rsp_dict, method_dict=method_dict)
rsp_results = rsp_drv.compute(molecule, basis, scf_results)
# Now we can analyze the densities.
# First, we have to define the subgroups. One subgroup will consist of the ethene molecule, while the other will consist of the tetrafluoroethene.
subgroup_name = ["E", "TFE"]
natm = molecule.number_of_atoms()
atom_subgroup_map = [-1] * natm
atom_subgroup_map[:6] = [0] * 6 # The first 6 atoms belong to ethene, subgroup 0
atom_subgroup_map[6:] = [1] * (natm-6) # The next 6 atoms belong to tetrafluoroethene, subgroup 1
print(atom_subgroup_map)
[0, 0, 0, 0, 0, 0, 1, 1, 1, 1, 1, 1]
def plot_transition_diagram(state_index, file_name, subgroup_name, atom_subgroup_map):
folder = "../../data/vis/" # Path to where the cube files were saved
density_type_plus = "detach" # or hole
density_type_minus = "attach" # or electron/particle
hole_cube = "%s_S%d_%s.cube" % (folder+file_name, state_index, density_type_plus)
particle_cube = "%s_S%d_%s.cube" % (folder+file_name, state_index, density_type_minus)
# We have to load the detachment and attachment densities and compute the atomic charges
transition = tau.load_transition(hole_cube, particle_cube)
segment_array = tau.compute_atomic_charges([transition])
subgroup_info = tau.SubgroupInfo()
# Determine subgroup charges
subgroup_info.set_subgroups(subgroup_name, atom_subgroup_map)
tau.compute_subgroup_charges(transition, subgroup_info)
parts = file_name.split('_')
diagram_title = "%s %s, S%d" % (parts[0], parts[1], state_index)
tau.create_diagram(subgroup_info, title=diagram_title,
save_plot=False)
plot_transition_diagram(1, 'dimer_4A', subgroup_name, atom_subgroup_map)
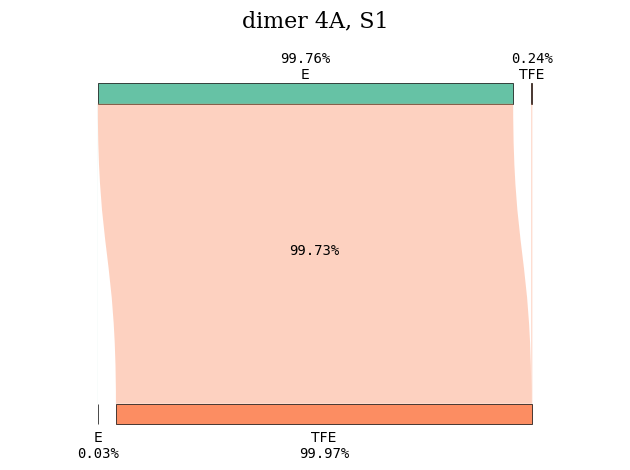
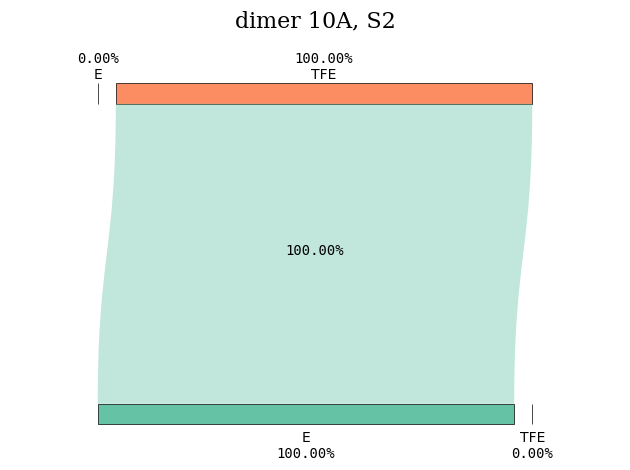
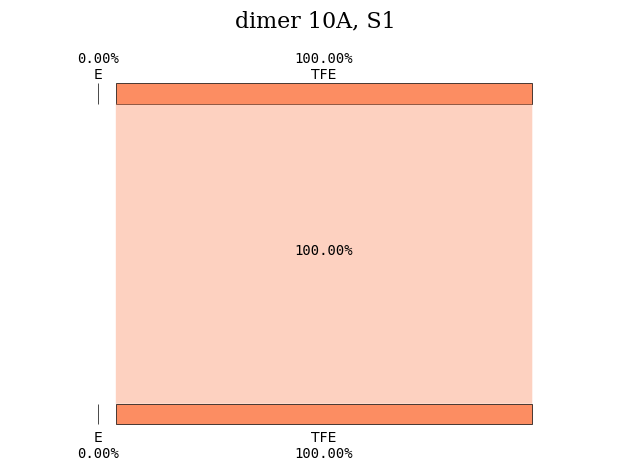
Repeating the same calculation for another intermolecular distance, we can compare what happens to the nature of the excited states. For an intermolecular distance of 10 \(\unicode{x212B}\), the first excited state is a local excitation involving the tetrafluoroethene subgroup.
plot_transition_diagram(1, 'dimer_10A', subgroup_name, atom_subgroup_map)
Instead, the first excited state with CT character is S\(_2\).
plot_transition_diagram(2, 'dimer_10A', subgroup_name, atom_subgroup_map)